How to read the Periodic Table
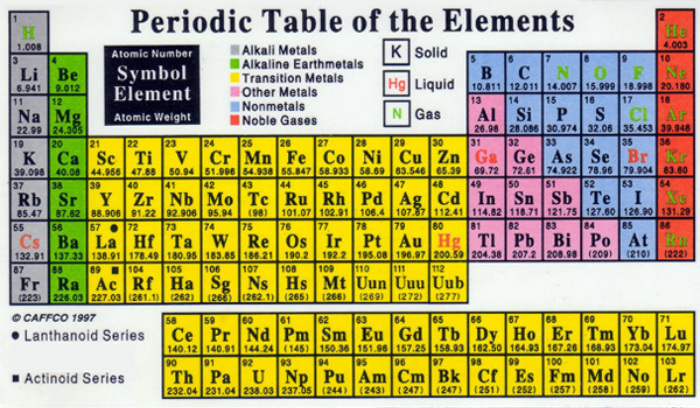
Don't be frightened by the periodic table. I know it looks very difficult to read and understand, but it really isn't. Here are the basics! The table is just a grid of all the elements in existence. These elements are arranged in order of their atomic numbers (the number of protons each atom has in its nucleus). The elements are arranged this way to keep elements of similar properties together. The periodic table has rows running left to right (which are called periods) and columns running up and down (which are called groups).
There are 18 groups (columns) in the periodic table. The elements are grouped together based on their similarities and because they all have the same number of electrons in their outer shell. By knowing which group the element is in you are able to tell a lot about the element.
The properties of the elements across a period (row) change gradually. There electron shells have the same numbers, but they are very different. When you move across the period (row), atoms begin to get heavier and smaller. This happens because the electron shells stay the same across the period but the number of protons in the nucleus gets larger, and the positively charged protons sucks the negatively charged electrons tighter into the center.
The colors of the periodic table represent the properties of each element. They are there to indicate whether an element is a solid, liquid or gas. For example: Liquids are written in red, gasses are the elements written in green, and most of the time elements written in black are solids.
There are 18 groups (columns) in the periodic table. The elements are grouped together based on their similarities and because they all have the same number of electrons in their outer shell. By knowing which group the element is in you are able to tell a lot about the element.
The properties of the elements across a period (row) change gradually. There electron shells have the same numbers, but they are very different. When you move across the period (row), atoms begin to get heavier and smaller. This happens because the electron shells stay the same across the period but the number of protons in the nucleus gets larger, and the positively charged protons sucks the negatively charged electrons tighter into the center.
The colors of the periodic table represent the properties of each element. They are there to indicate whether an element is a solid, liquid or gas. For example: Liquids are written in red, gasses are the elements written in green, and most of the time elements written in black are solids.
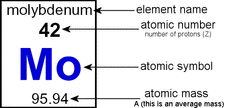
As you can see on the image to the left, the element is named (molybdenum) followed by the number below. This is the atomic number. The atomic number refers to the number of protons in the element. The Mo under the atomic number is the atomic symbol. This symbol tells us what the element is (rather than using the element's name). Under the symbol is the number telling the atomic mass of the element. The atomic mass is the total mass of protons, neutrons, and electrons in a single atom.
If you would like to learn more about how the read the periodic table, I found a good website that you can use to have a better understanding of it. Click on the link below: (to open highlight the link, right click, choose open link in new tab) .
https://www.webelements.com/manganese/
https://www.webelements.com/manganese/
